
File on 4 investigations
 BBC
BBCIt was known that the transplantation of knee replacement, used in thousands of UK operations, had an eight -year failure rate before it was withdrawn, and the British Broadcasting Corporation was discovered.
The patients told a file on 4 investigations on how to leave them as moving or addicted to pain relievers after receiving the Nexgen knee transplantation, because it ended up getting out of its place. Hundreds of people have now had a second corrective process.
Knee surgeons says that the American agricultural manufacturer, Zimmer Biomett, took a long time to admit that there is a problem with one certain component.
Zimmer Biomett says that patient safety is “a top priority” and that their products are approved according to relevant regulations.
Deby Baker of Southampton had a process to replace her left knee in 2016.
Although she was initially successful, she started suffering from severe pain after a year during a holiday at Majorca.
“I put a bag of ice on my knees for four days, I had to do this every few hours because I was in a state of torment,” she says.
The replacement of the knee includes removing the damaged surfaces from the thigh bone (thigh bone) and the tube (Xin bone) and replacing it with artificial ingredients.
Deby says the pain has caused knee planting from ticks and bone wearing.
During the next few months, she says she has become a prescription pain reliever: “I was in fentian and morphine. It took a long time to get out of the morphine because I am addicted.”
Since then, she has had a second alternative to the knee, but the problems caused by the first failed planting have caused long -term health problems, she says.
“I put my whole body from alignment, I walk with a lame,” Deby says. As a result, you are waiting now to replace the hip.
Another patient, “Diana” (and not her real name), had a knee transplant equipped in 2021, which also retreated and began to wear her extreme bone, leaving her almost non -moving.
“The consultant told me every time I stand, I was standing on a broken leg. It was absolute suffering,” she says.
Diana asked to be unknown because she was working in NHS.
As part of the knee alternatives, DEBIE and Diana received a specific section of the transplant, known as the “ingredient component”, also known as “Al -Dabboubi tray”.
In broad phrases, this section was lacking in a layer of plastic contained in the previously respected versions of the Nexgen knee.
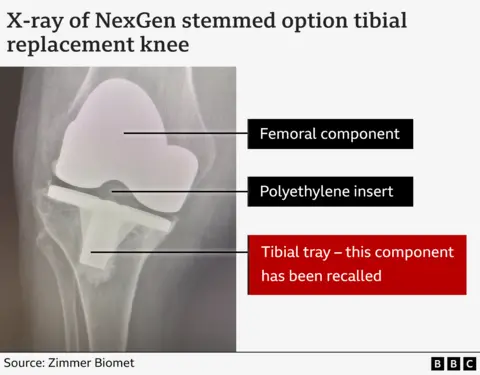
Zimmer Biomett started marketing this modified version in 2012. It was cheaper than the previous model, so it was the financial logical of NHS, according to Professor David Barrett, a knee specialist at Southampton University.
“[The NHS] He was justified by saying: “We have all the reasons for the belief that it will be fine,” he says.
In the following contract, more than 10,000 patients were provided with this version of the transplant.
However, a file discovered 4 investigations that concerns were first placed in 2014 by the National Joint Register (NJR), which maintains a transplant surgery record throughout England, Wales and Northern Ireland.
At this point, there was not enough data to draw any reliable conclusions, NJR told us. He added that it is not an easy task to isolate a specific component that does not work as it should.
More concerns about the transplant in Ireland arose two years later, in 2016, by Professor Eric Masterson, knee surgeon in Limric.
Professor Mastercson’s corrective surgery increased after the NEXGEN implants began in 2012 and found that his professional competence was questioned.
“This was a single place,” says a file in 4 investigations. “It spends a lifelong building a profession and a reputation, and it is very easy to tear this profession.”
When I raised questions with Zimmer’s representatives, they assured him that there was no widespread problem, he says – an account that NHS surgeons who told us that they found themselves in similar situations.

Professor Masterson requested to communicate with the UK surgeons to compare notes. However, the company’s secret internal documents seen on a file on 4 investigations reveal that the company was only ready to contact the surgeons on his behalf if they consider “friends from Zimmer Biomett” and “Happy with their patients in Nexgen”.
Zimmer Biomett has failed to act quickly after determining the problem, according to Professor Leela Bayanti, a UK’s top knee surgeon. She says fears have raised herself and other colleagues until 2017.
“The case is [the company’s] The initial frequency in recognizing a problem and not dealing with an even evaluation of these patients [Zimmer Biomet] She told us, “I reached a position they had.”

In 2022, NJR estimated that patients were likely to need almost corrective surgery after receiving Nexgen transplantation, compared to average knee planting.
In the same year, Zimmer Biomett called any unused cultivation from the UK market.
The failure rates of the failure of the ingredient ingredient in this Nexgen transplantation varies from 6 % (twice what should be expected) to 19 %, according to Academic studies reviewed by the peers.
In a statement, the company told the BBC: “Zimmer Biomett is committed to the highest standards of patient safety, quality and transparency. When the new data becomes available, we behave appropriately, responsibly and according to the applicable regulatory requirements.”
All 10,000 patients with problematic cultivation must now be called for a review by hospitals as they had their initial operations. Hundreds have already had to do a second operation, and others could follow with problems.
The cost of correcting the problem is not cheap. Professor Barrett of Southampton University says every review costs between 10,000 pounds and 30,000 pounds because the planting is very specialized.
“Patients in the hospital for a much longer period require more support. So this is very important expenses,” he says.
As a result, the total bill is estimated to collide with millions of pounds.
Zimmer Bionet did not respond when a file was asked about 4 investigations if it would contribute to the cost of these operations. However, we have seen a secret email message to a company, which was sent in 2022, and I asked sales staff to say that “Zimmer Biometst will not cover the costs of diagnosis, follow -up or review at the forefront.”
NHS England told us that it is “currently retracting the case that implants the Zimmer Biometh Nexgen”.

